Chemistry is the
science of the composition, structure, properties and reactions of matter,
especially of atomic and molecular systems. Medicines or drugs that we take for
the treatment of various ailments are chemicals, either organic or inorganic. However,
most drugs are organic molecules. Let us take aspirin as an example. It is
probably the most popular and widely used analgesic drug because of its
structural simplicity and low cost. Aspirin is chemically known as acetyl
salicylic acid, an organic molecule. The precursor of aspirin is salicin, which
is found in willow tree bark.
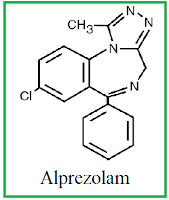
Anxiety Drugs
Anti-anxiety drugs, also known as tranquilizers, are
medications that relieve anxiety by slowing down the central nervous system.
Their relaxing and calming effects have made them very popular: anti-anxiety
drugs are the most widely prescribed type of medication for anxiety. They are
also prescribed as sleeping pills and muscle relaxants. Benzodiazepines
are the most common class of anti-anxiety drugs. They include:
Antihistamine
Antihistamine, any of a
group of synthetic drugs that selectively counteract the pharmacological
effects of histamine, following its release from certain large cells (mast
cells) within the body. Antihistamines replace histamine at one or the other of
the two receptor sites at which it becomes bound in various susceptible
tissues, thereby preventing histamine-triggered reactions under conditions such
as stress, inflammation, and allergy. Antihistamines with powerful antiemetic
properties are used in the treatment of motion sickness and vomiting. The most
common examples are:
Painkillers
Prescription
painkillers are powerful drugs that interfere with the nervous system’s
transmission of the nerve signals we perceive as pain. Most painkillers also
stimulate portions of the brain associated with pleasure. Thus, in addition to
blocking pain, they produce a “high.”
The most powerful
prescription painkillers are called opioids, which are opium-like compounds.
They are manufactured to react on the nervous system in the same way as drugs
derived from the opium poppy, like heroin. The most commonly abused opioid
painkillers include:
Antobiotics
Antibiotics or
antibacterials are a type of antimicrobial used in the treatment and prevention
of bacterial infection. They may either
kill or inhibit the growth of bacteria. Several antibiotics are also effective
against fungi and protozoans, and some are toxic to humans and animals, even
when given in therapeutic dosage. Antibiotics are not effective against viruses
such as the common cold or influenza, and may be harmful when taken
inappropriately.
The overwhelming
majority of antibiotics are made from living organisms such as bacteria about
90% of antibiotics are isolated from bacteria fungi, and molds. Others are
produced synthetically, either in whole or in part.
At one time all
antibiotics were made from living organisms. This process, known as biosynthesis,
is still used in the manufacture of a number of antibiotics. In this method, it
is actually the organisms themselves that manufacture the antibiotic. The
laboratory technician merely provides favorable conditions for the organisms to
multiply, and then extracts the drug. For example, mold organisms are placed in
a medium (a substance used for the growth of microorganisms) such as corn
liquor to which milk sugar has been added. This mixture forms a liquid that is
put into a tank, which is kept at a temperature of25 degrees Centigrade (77
degrees Fahrenheit) and shaken for over 100 hours.The mold organisms multiply
rapidly in this warm liquid, producing penicillinin the process.
All types of penicillin
have an identical ring. However, in each type of penicillin, the chemical chain
attached to the ring is different. By modifying the molecules of the chain,
scientists are able to create drugs with a wide range of effects on a variety
of organisms. Some of these drugs are useful in treating infections.
Pharmaceutical
companies use computer-generated images of the rings and experiment with a
countless variety of possible chains. Researchers have developed antibiotics
with long half-lives (period of effectiveness), which means that the medication
can be taken every 24 hours instead of every few hours. The newer antibiotics
are also more effective against a wider range of infections than were earlier
drugs. Common examples are:
 |
| Penicillin |
 | |||
| Ampicillin |
Vitamins
Vitamins are organic
compounds which are needed in small quantities to sustain life. We get vitamins
from food, because the human body either does not produce enough of them, or
none at all.
 |
| Vitamin - A |
An organic compound
contains carbon. When an organism (living thing) cannot produce enough of an
organic chemical compound that it needs in tiny amounts, and has to get it from
food, it is called a vitamin.
 | |
| Vitamin - C |
Sometimes the compound
is a vitamin for a human but not for some other animals. For example, vitamin C
(ascorbic acid) is a vitamin for humans but not for dogs, because dogs can
produce (synthesize) enough for their own needs, while humans cannot.
 | |
| Vitamin - D |
Vitamins can be derived
from plant or animal products, or produced synthetically in a laboratory.
Vitamin A, for example, can be derived from fish liver oil, and vitamin C from
citrus fruits or rose hips. Most commercial vitamins are made from synthetic
vitamins, which are cheaper and easier to produce than natural derivatives. So
vitamin A may be synthesized from acetone, and vitamin C from keto acid. There
is no chemical difference between the purified vitamins derived from plant or
animal sources and those produced synthetically. Different laboratories may use
different techniques to produce synthetic vitamins, as many can be derived from
various chemical reactions.
 |
| Vitamin -E |
Vitamin tablets or
capsules usually contain additives that aid in the manufacturing process or in
how the vitamin pill is accepted by the body. Microcrystalline cellulose,
lactose, calcium, or malto-dextrin are added to many vitamins as a filler, to
give the vitamin the proper bulk. Magnesium stearate or stearic acid is usually
added to vitamin tablets as a lubricant, and silicon dioxide as a flow agent.
These additives help the vitamin powder run smoothly through the tablet-making
or encapsulating machine. Modified cellulose gum or starch is often added to
vitamins as a disintegration agent. That is, it helps the vitamin compound
break up once it is ingested. Vitamin tablets are also usually coated, to give
the tablets a particular color or flavor, or to determine how the tablet is
absorbed (in the stomach versus in the intestine, slowly versus all at once,
etc.). Many coatings are made from a cellulose base. An additional coating of
carnauba wax is often put on as well, to give the tablet a polished appearance.
 |
| Vitamin - K |
Herbs of various kinds
may be added to vitamin compounds, as well as minerals such as calcium, iron,
and zinc. Typically, specialized laboratories produce purified vitamins and
minerals. A distributor buys these from the laboratories and sells them to
manufacturers, who put them together in different compounds such as
multivitamin tablets or B-complex capsules.
Vitamin B complex
|
Vitamin
|
Naame
|
Structure
|
Molecular
Funnction
|
|
B1
|
Thiamine
|
|
Thiamine
plays a central role in the generation of energy from carbohydrates. It is
involved in RNA and DNA production, as well as nerve function. Its active
form is a coenzyme called thiamine pyrophosphate (TPP), which takes part in
the conversion of pyruvate to acetyl coenzyme A (CoA) in metabolism.
|
|
B2
|
Riboflavin
|
|
Riboflavin
is involved in the energy production for the electron transport chain, the
citric acid cycle, as well as the catabolism of fatty acids (beta oxidation)
|
|
B3
|
Niacin
|
|
Niacin
is composed of two structures: nicotinic acid and nicotinamide. There are two
co-enzyme forms of niacin: nicotinamide adenine dinucleotide (NAD) and
nicotinamide adenine dinucleotide phosphate (NADP). Both play an important
role in energy transfer reactions in the metabolism of glucose, fat and
alcohol.
NAD
carries hydrogens and their electrons during metabolic reactions, including
the pathway from the citric acid cycle to the electron transport chain. NADP
is a coenzyme in lipid and nucleic acid synthesis.
|
|
B5
|
Pantothenic
Acid
|
|
Pantothenic
acid is involved in the oxidation of fatty acids and carbohydrates. Coenzyme
A, which can be synthesised from pantothenic acid, is involved in the
synthesis of amino acids, fatty acids, ketones, cholesterol, phospholipids,
steroid hormones, neurotransmitters (such as acetylcholine), and antibodies.
|
|
B6
|
pyridoxine,
pyridoxal, pyridoxamine
|
|
The
active form pyridoxal 5'-phosphate (PLP) (depicted) serves as a cofactor in
many enzyme reactions mainly in amino acid metabolism including biosynthesis
of neurotransmitters.
|
|
B7
|
Biotin
|
|
Biotin
plays a key role in the metabolism of lipids, proteins and carbohydrates. It
is a critical co-enzyme of four carboxylases: acetyl CoA carboxylase, which
is involved in the synthesis of fatty acids from acetate; propionyl CoA
carboxylase, involved in gluconeogenesis; β-methylcrotonyl CoA carboxylase, involved in the
metabolism of leucine; and pyruvate CoA carboxylase, which is involved in the
metabolism of energy, amino acids and cholesterol.
|
|
B9
|
Folic
Acid
|
|
Folic
acid acts as a co-enzyme in the form of tetrahydrofolate (THF), which is
involved in the transfer of single-carbon units in the metabolism of nucleic
acids and amino acids. THF is involved in pyrimidine nucleotide synthesis, so
is needed for normal cell division, especially during pregnancy and infancy,
which are times of rapid growth. Folate also aids in erythropoiesis, the
production of red blood cells.
|
|
B12
|
Cobalamin
|
|
Vitamin
B12 is involved in the cellular metabolism of carbohydrates, proteins and
lipids. It is essential in the production of blood cells in bone marrow, and
for nerve sheaths and proteins. Vitamin B12 functions as a co-enzyme in
intermediary metabolism for the methionine synthase reaction with
methylcobalamin, and the methylmalonyl CoA mutase reaction with
adenosylcobalamin.
|










-Pantothenic_acid_Formula_V.1.svg/405px-(R)-Pantothenic_acid_Formula_V.1.svg.png)



No comments:
Post a Comment